Novel MOA
Current antipsychotic treatments rely on the same primary mechanism of action (MOA) as they did when the first antipsychotic was discovered in the 1950s: inhibiting D2 dopamine receptors. Current antipsychotics are often used by physicians to address a wide range of neuropsychiatric disorders in addition to schizophrenia, including bipolar disorder and psychotic depression, as well as psychosis and agitation in elderly patients with dementia, but are associated with modest efficacy and significant side effects.
Muscarinic receptor agonists emerged in the 1990s as a promising innovative approach for treating psychosis and cognitive impairment. Muscarinic receptors are g-protein linked receptors (GPCRs) that bind the neurotransmitter acetylcholine. There are five distinct muscarinic receptors, M1-M5, found in the brain as well as various peripheral tissues.
The link between muscarinic receptor stimulation in the CNS, particularly stimulation of M1 and M4 receptors, and the reduction of psychotic symptoms and cognitive impairment, has been well studied and is supported by data from preclinical studies and randomized, double-blind, placebo-controlled clinical trials with xanomeline published in peer reviewed journals. However, the successful development of a therapeutic agent targeting muscarinic receptors has been limited by undesirable side effects that are believed to arise primarily as a result of stimulation of muscarinic receptors in peripheral tissues.
We believe a therapeutic agent that can preferentially target and stimulate muscarinic receptors in the CNS, but not in peripheral tissues, has the potential to treat psychosis in schizophrenia and AD, including the associated agitation in patients with AD. We also believe the preferential stimulation of M1 and M4 muscarinic receptors in the CNS may address the negative symptoms of schizophrenia, such as apathy, reduced social drive and loss of motivation, as well as cognitive deficits in working memory and attention, all of which currently lack any approved treatments. This approach has the potential to produce a differentiated therapy relative to current D2 dopamine receptor-based antipsychotic drugs and to beneficially impact the lives of millions of patients with schizophrenia and other psychotic and cognitive disorders.
KARXT
KarXT combines xanomeline, a muscarinic receptor agonist that preferentially stimulates M1 and M4 muscarinic receptors, and trospium, an approved muscarinic receptor antagonist that does not measurably cross the blood-brain barrier, confining its effects to peripheral tissues. M1 and M4 muscarinic receptors are the receptor subtypes believed to mediate the antipsychotic, procognitive and analgesic effects of xanomeline and other muscarinic agonists. Results from preclinical studies and clinical trials conducted by third parties support the hypothesis that xanomeline can reduce psychosis and improve cognition. Like all muscarinic receptor agonists studied to date, however, xanomeline’s tolerability has been limited by side effects arising from muscarinic receptor stimulation in peripheral tissues, leading to nausea, vomiting, diarrhea and increased salivation and sweating, collectively referred to as cholinergic adverse events. Trospium is a muscarinic receptor antagonist approved in the United States and Europe for the treatment of overactive bladder that inhibits all five muscarinic receptor subtypes in peripheral tissues.
We believe that the combination of xanomeline, a centrally- acting muscarinic agonist, and trospium, a peripherally-acting muscarinic antagonist, will have the therapeutic benefits of xanomeline but with markedly reduced side effects.
We assessed the potential of over 7,000 possible combinations of muscarinic receptor agonists and antagonists to find an optimized combination that could preferentially stimulate muscarinic receptors in the CNS to improve the symptoms of psychosis, while avoiding stimulation of muscarinic receptors in the peripheral tissues and the associated side effects. As a result of our research, we identified xanomeline and trospium as the most promising pairing for development in the form of KarXT (Karuna-xanomeline-trospium). Trospium is a potent and effective muscarinic receptor antagonist that does not measurably cross the blood-brain barrier, confining its effects to peripheral tissues, and it currently marketed for the treatment of overactive bladder in the US and other territories worldwide. Karuna co-founder and chief operating officer, Andrew Miller, PhD, identified the initial hypothesis and driving the execution of the completed Phase I studies supporting the combination of xanomeline and trospium.
Originally developed by Eli Lilly, xanomeline as a treatment for psychosis and related neuropsychiatric disorders has been examined in clinicals trials enrolling over 800 subjects or patients conducted by us and third parties, with 68 patients being dosed for at least one year and a maximum treatment duration of almost four years. Xanomeline has demonstrated efficacy in reducing psychosis and improving cognition in placebo-controlled human trials in both Alzheimer’s disease and schizophrenia.
A randomized, double-blind, placebo-controlled, small Phase 2 trial of xanomeline was conducted in 20 patients with schizophrenia with acute psychosis, as a collaboration between Eli Lilly and the Indiana University School of Medicine. This monotherapy trial used the Positive and Negative Syndrome Scale, or PANSS, as a primary endpoint. The PANSS is a set of measurements used for evaluating symptom severity in patients with schizophrenia and the change in PANSS score has been used as the primary endpoint in many registrational trials of antipsychotic medicines. A clinically meaningful and statistically significant 24-point PANSS score difference was observed between xanomeline and placebo was observed. By comparison, meta-analyses of published clinicals trials of currently approved antipsychotic medicines report an average difference of nine to ten points in PANSS score versus placebo. Historically, changes as small as five points have supported the approval of current antipsychotics. Significant benefits on multiple cognitive tests were also observed in the xanomeline treatment group vs. placebo. This data was published by Shekhar et al.
In a randomized, double-blind, placebo controlled Phase II study in 343 Alzheimer's disease patients, patients treated with xanomeline were observed to have dose-dependent decreases in multiple psychotic symptoms and related behaviors, including hallucinations, delusions and agitation, as compared to patients on placebo. These responses were seen as early as two to three weeks after commencement of dosing with xanomeline. Xanomeline was also observed to reduce the emergence of psychotic symptoms over the course of the six-month trial in patients who did not have psychotic symptoms at the initiation of the trial.
Despite xanomeline’s promising therapeutic benefit in treating psychosis and related behavioral symptoms in patients with schizophrenia and AD, its potential has been limited by cholinergic side effects, which are believed to result from the stimulation of muscarinic receptors in peripheral tissues.
We believe that the above data and our phase I clinical data with KarXT demonstrated significant reductions in the adverse events associated with xanomeline and the well tolerated nature of KarXT at xanomeline doses equivalent or higher than previously tested in Phase 2, support the further development of KarXT in multiple CNS disorders, including the treatment of the positive, negative, and cognitive symptoms of schizophrenia, psychosis and agitation associated with dementia, including AD, and as a novel non-opioid therapeutic for various forms of post-operative, inflammatory and neuropathic pain.
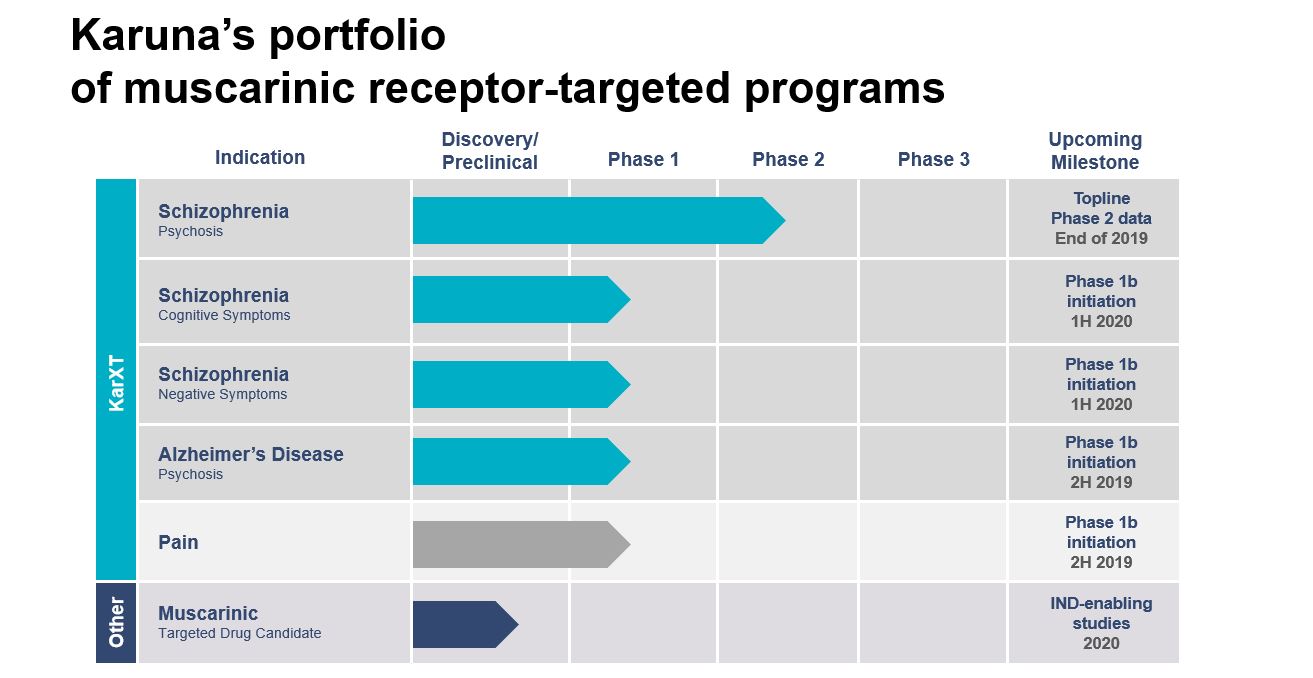
Pipeline
Our pipeline is built on the broad therapeutic potential of our lead product candidate, KarXT, an oral modulator of muscarinic receptors that are located both in the central nervous system (CNS) and various peripheral tissues. KarXT is our proprietary product candidate that combines xanomeline, a novel muscarinic agonist, with trospium, an approved muscarinic antagonist, to preferentially stimulate muscarinic receptors in the CNS.
Selected Xanomeline receptor Research
Clinical:
Shekhar A, Potter WZ, et al. Selective Muscarinic Receptor Agonist Xanomeline as a Novel Treatment Approach for Schizophrenia. American Journal of Psychiatry 2008; 165:1033–1039.
Bodick NC, Offen WW, Levey AI, et al. Effects of Xanomeline, a Selective Muscarinic Receptor Agonist, on Cognitive Function and Behavioral Symptoms in Alzheimer Disease. Arch Neurol. 1997; 54(4): 465–473.
Farde, L., Suhara, T. et al: PET Study of the M1-Agonists [11C]Xanomeline and [11C]Butylthio-TZTP in Monkey and Man. Dementia and Geriatric Cognitive Disorders, 1996; 7(4), 187-195.
Preclinical:
Barak S, Weiner I: The M1/M4 preferring agonist xanomeline reverses amphetamine-, MK801- and scopolamine-induced abnormalities of latent inhibition: putative efficacy against positive, negative and cognitive symptoms in schizophrenia. International Journal of Neuropsychopharmacology 2011; 14:1233–1246.
Shannon HE, Rasmussen K, et al: Xanomeline, an M1/M4 preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophrenia Research 2000; 42: 249–259.
Thorn C, Moon J et al. Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor Mediated Effects of Xanomeline. ACS Chemical Neuroscience 2019 10 (3), 1753-1764.
Pain:
Martino G, Puma C, et al. The M1/M4 preferring agonist xanomeline is analgesic in rodent models of chronic inflammatory and neuropathic pain via central site of action. Pain 2011; 152:2852–2860.
Naser PV, Kuner R. Molecular, cellular and circuit basis of cholinergic modulation of pain. Neuroscience. 2017; S0306-4522(17)30625-5
Sheardown MJ, et al. M1 receptor agonist activity is not a requirement for muscarinic antinociception.J Pharmacol Exp Ther. 1997 May;281(2):868-75.
Wess J et al. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: a review. Life Sci. 2003;72(18-19):2047-54.